
New Drug Designations - July 2023
Shots:
-
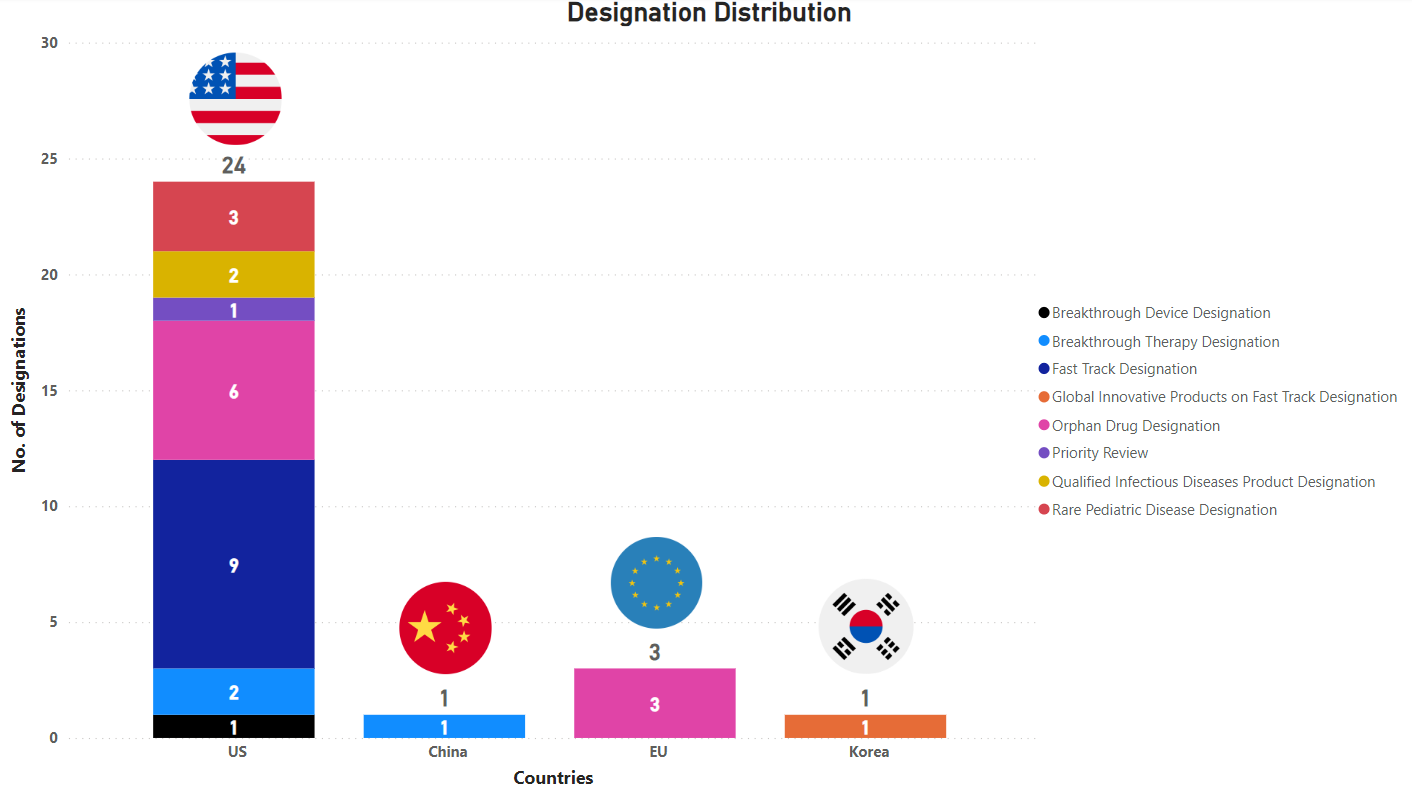
PharmaShots' designation report provides a concise overview of several drugs and their designations by the US FDA, the EU, China, and Korea. This month’s report includes 7 biological drugs, 10 small molecules, 8 cell and gene therapies, 1 radiopharmaceutical, 2 microbiota and 1 diagnostic test
-
MyoPax’ Satori-01, focused on repairing the Exstrophy-Epispadias Complex sphincter defect, is the drug to receive both RPDD and ODD from the US FDA
-
PharmaShots has compiled a list of a total of 29 drugs awarded with designations by multiple regulatory bodies in Jul 2023

Satori-01

-
The P-I/IIa FIH (MuST) study evaluates the safety and efficacy of Satori-01 and focuses at repairing the EEC sphincter defect in Germany
-
The trial is sponsored by the Charité Universitätsmedizin and financed by the German Federal Ministry of Education and Research and the ForTra GmbH of the Else-Kröner-Fresenius Foundation under the guidance of Prof. Simone Spuler. Prof. Wolfgang Rösch
SNB-101

-
The company’s SNB-101, the world's first nanoparticle anticancer drug, is approved for P-I study in the US (NCT04640480) and Korea. The IND application has been filed for P-II study in Korea
-
SNB-101 received ODD based on the preclinical efficacy results in small-cell lung cancer models. Also, it is expected to be effective for pancreatic cancer, and stomach cancer as well.
Padeliporfin VTP

-
The IND submission for P-I trial of padeliporfin VTP for the treatment of patients with locally advanced pancreatic ductal adenocarcinoma is planned and expected to enroll in 2023
-
Earlier, Padeliporfin VTP has been granted with FTD and ODD from the US FDA for the treatment of adult patients with low-grade upper ract urothelial carcinoma (UTUC)
-
Padeliporfin VTP (Vascular Targeted Photodynamic therapy) is a minimally invasive oncology platform offering surgery-like efficacy combined with healthy-tissue or organ preservation in solid tumors for high-risk surgical patients with unmet needs where surgery is not the preferred clinical option, or the risk of surgery is too high
Gallium Maltolate (GaM)

-
The pre-clinical study of gallium maltolate (oral) for pediatric GBM showed significant survival benefit and was presented at SNO Pediatric Conference 2023
-
Earlier, in Feb the company also received ODD for adult GBM from the US FDA
-
The results showed m-OS of more than 2x longer in the group treated with GaM vs the untreated controls
IBP-1016

-
The US FDA has granted ODD for gastroschisis which can result in growth retardation, sepsis and NEC leading to increased mortality
ISB 2001

-
Ichnos plans to initiate P-I FIH dose-escalation and expansion trial evaluating ISB 2001, after securing approval from the HREC in Australia and IND clearance from the US FDA
-
ISB 2001 is BCMA x CD38 x CD3 TREAT1 trispecific antibody based on the proprietary BEAT platform enabling the development of immune cell engagers
Taldefgrobep Alfa (BHV2000)

-
Biohaven is currently evaluating taldefgrobep alfa (BHV2000) in the P-III (RESILIENT) trial for its efficacy and safety in patients with spinal muscular atrophy
-
Earlier, the company also received ODD and FTD from the US FDA for SMA
-
Taldefgrobep alfa is a novel anti-myostatin adnectin having the potential to treat SMA by blocking myostatin, when used in combination with currently available disease modifying therapies to preserve motor neurons
Nomacopan

-
Akari is currently conducting a registrational P-III trial evaluating nomacopan in pediatric hematopoietic stem cell transplant-related thrombotic microangiopathy (HSCT-TMA)
-
In 2024, the company plans to initiate the P-III double-blind PBO-controlled study of nomacopan in adult HSCT-TMA
-
Nomacopan has also received RPD (which entitles Akari to a Priority Review Voucher at approval), ODD (pediatric and adult), and FTD (pediatric) for HSCT-TMA by the US FDA
MT-401

-
The P-II study evaluates MT-401 for the treatment of relapsed AML following allogeneic HSCT and was developed to target 4 different antigens that are upregulated in AML but have limited expression on normal cells
-
MT-401 employs a novel non-genetically modified approach that recognizes multiple antigens expressed on tumor cells and helps in minimizing tumor escape

Selinexor

-
The P-III (XPORT-MF-034) study, initiated in Jun 2023, is to evaluate the efficacy and safety of selinexor (60mg, qw) + ruxolitinib in JAKi-naïve patients with myelofibrosis. The top-line data is anticipated in 2025
-
As the data cutoff of Apr 2023, the updated results from the P-I (XPORT-MF-034) trial highlighted rapid, deep and sustained spleen responses and robust symptom improvement in patients and were presented at the AACR, ASCO and EHA 2023 annual meeting
-
The company plans to evaluate selinexor in combinations thus expanding its clinical development program for myelofibrosis in other JAKi-naïve settings
Labafenogene Marselecobac (SYNB1934)

-
Labafenogene marselecobac has previously received RPDD and ODD by the US FDA and ODD from the EMA
-
The P-II study results have shown the potential of labafenogene marselecobac as the first therapy for PKU that is expected to be approved as both monotx. and adjunctive medical treatment. With these successful results, labafenogene marselecobac will be evaluated in global, pivotal P-III (Synpheny-3) trial
-
Labafenogene marselecobac (formerly SYNB1934) is a non-systemically absorbed, potential treatment for phenylketonuria (PKU) which is administered orally
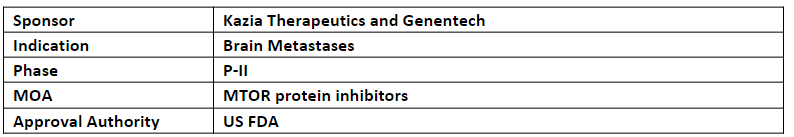
Paxalisib
-
The FTD has been granted based on the promising results from an interim analysis of an ongoing P-I study of paxalisib + WBRT for brain metastases from a primary tumor that showed a 100% (n=9) response. The results from stage 1 were presented at CNS Clinical Trials and Brain Metastases annual meeting 2022, organized jointly by SNO and ASCO
-
The expansion P-I study includes 2 stages in which 1st stage aims to establish MTD in 12 patients and 2nd stage aims to evaluate the efficacy for further development of paxalisib + WBRT
-
Paxalisib is being evaluated in 9 ongoing studies incl. ongoing P-I trial whose data is anticipated in Q1’24, the final data from the (GBM AGILE) trial of paxalisib for glioblastoma is anticipated in H2’23 and the initial data from the P-II trial of paxalisib pediatric patients with diffuse midline gliomas is anticipated in Q3’23
-
Paxalisib has received ODD and FTD in Feb 2018 and Aug 2020 for glioblastoma respectively, RPD and ODD for diffuse intrinsic pontine glioma in Aug’20 and RPD and ODD for atypical teratoid/rhabdoid tumors (AT/RT) in Jun 2022 and Jul 2022, respectively
STAR-0215

-
The P-IBM/II trial (ALPHA-STAR) evaluates STAR-0215 in patients with hereditary angioedema. The initial PoC results are anticipated in mid-2024
-
STAR-0215 (Q3M/Q6M) is a monoclonal antibody being developed for the preventive treatment of HAE and improve the quality of life
Remoras

-
Generex will enroll its 1st patient in P-I/II dose escalation study (Acclaim-3) in Q3’23 to evaluate Remoras + TelCentris for patients who did not develop tumor progression after receiving Tecentriq and CT as initial standard treatment
-
Reqorsa has previously received FTD in combination with AstraZeneca's Tagrisso and with Merck’s Keytruda
IVS-3001

-
The FTD was based on the data of the IND submission in patients with HLA-G+ locally advanced or metastatic clear cell renal cell carcinoma (RCC) who have failed or are intolerant to standard RCC therapies
-
IVS-3001 is a CAR-T cell immunotherapy targeting the rarely exploited immune checkpoint and tumor-specific antigen known as HLA-G
EBT-101

-
EBT-101 is being evaluated in an ongoing P-I/II study for its safety and tolerability in adults (18 to 65yrs.) with HIV-1 who are on continuous antiretroviral therapy with HIV RNA below the level of detection
-
The in vivo CRISPR-based therapeutic, EBT-101, is designed to cure HIV infection after a single IV infusion
ARX517

-
ARX517 is currently being evaluated in the P-I/II (APEX-01) open-label dose escalation and dose expansion trial for patients with mCRPC whose tumors have progressed on two prior FDA-approved treatments for prostate cancer
-
The therapy is designed to promote highly specific tumor cell killings with minimal off-target toxicity
-
The company plans to present updated preliminary data from APEX-01 at a major medical meeting. ARX517 was recently featured in Nature
BRG01

-
Apart from FTD, earlier BRG01 has received ODD in Jun 2023 and IND approvals from the NMPA and the US FDA in Dec 2022 and Feb 2023 respectively
-
BRG01 is an engineered T cell therapy for nasopharyngeal cancer treatment

RBT-1

-
Along with this BTD, the company confirmed its P-III pivotal study for RBT-1
-
BTD was granted based on results from the P-II study that achieved the primary biomarker endpoint and other key clinical outcome endpoints supporting RBT-1 to improve postoperative outcomes in cardiothoracic surgery
-
Although the designation was granted in Jun 2023, but the company published it in Jul 2023 with its FDA’s agreement on the P-III program for RBT-1
Zenocutuzumab

-
This 2nd BTD has been granted based on the results from the ongoing P-I/II (eNRGy) study Early Access Program (EAP) evaluating safety and anti-tumor activity of zenocutuzumab monotx. in NRG1+ cancer. The data was presented in ASCO 2021 and 2022 annual meetings
-
The company is also evaluating zenocutuzumab + ADT (enzalutamide or abiraterone) in CRPC, irrespective of NRG1+ status and afatinib in patients with NRG1+ NSCLC. The initial data in CRPC is anticipated this year
Fruquintinib

-
The NMPA has granted BTD to the combination of fruquintinib and sintilimab for the treatment of patients with advanced EMC with pMMR1 tumors that have failed one line of Pt-based therapy
-
A total of 142 previously treated, advanced EMC patients were enrolled in the study of fruquintinib + sintilimab having 1EPs of IRC assessed ORR while the 2EPs of DCR, PFS, OS and PK assessments. Positive outcomes could lead to the NMPA submission in H1’24
-
The therapy was approved for marketing in China in Sep 2018 and commercially launched in Nov 2018 under the brand name Elunate for metastatic CRC patients

Zolbetuximab

-
The priority review for the BLA was based on results from the P-III (SPOTLIGHT) and (GLOW) studies evaluating zolbetuximab + mFOLFOX6 vs PBO + mFOLFOX6 and zolbetuximab + CAPOX vs PBO + CAPOX respectively. The PDUFA date has been set for Jan 12, 2024
-
The studies identified 38% of patients with CLDN18.2+ tumors as determined by a validated IHC assay
-
Zolbetuximab is a FIC investigational CLDN18.2-targeted mAb developed for 1L treatment of patients with locally advanced unresectable or metastatic HER2- G/GEJ adenocarcinoma whose tumors are CLDN18.2+

Retinol Palmitate

-
The designation has been granted for metabolic and reparative respiratory drugs for the prevention of bronchopulmonary dysplasia (BPD) in premature infants
-
The company expects PDUFA Meeting late-2023 with the US FDA to establish pathway for US approval of retinol palmitate
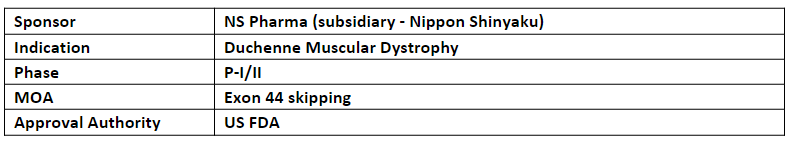
Brogidirsen (NS-089/NCNP-02)
-
The P-II trial of NS-089/NCNP-02 is planned in the US and Japan by NS Pharma and Nippon Shinyaku respectively
-
NS-089/NCNP-02 (an antisense nucleotide) is developed through joint research between Nippon Shinyaku and the National Center for Psychiatry and Neurological Medicine
Satori-01

-
The clinical study (NCT04729582) for EEC will begin in Germany under the sponsorship and funding from Charité – Universitätsmedizin Berlin and German Federal Ministry of Education and Research and the ForTra gGmbH of the Else-Kröner-Fresenius Foundation respectively
-
The company also plans to expand the study in the US

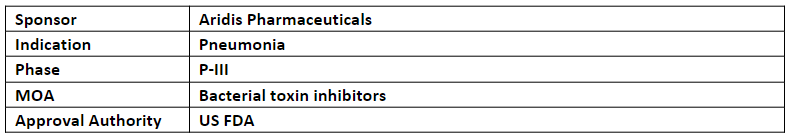
AR-301
-
The AR-301 (human IgG1 mAb) is currently being evaluated in P-III study as an adjunctive therapy for ventilator associated pneumonia (VAP), ventilated hospital acquired pneumonia (HAP), and ventilated community acquired pneumonia (CAP) patients
CSA-131

-
The QIDP designation has been granted to CSA-131 for the treatment of life-threatening Pseudomonas infections in patients with Cystic Fibrosis
-
CSA-131 is a synthetic non-peptide mimic of the endogenous antimicrobial peptide LL-37, essential for preventing and treating lung infections

Lu-177-DGUL

-
The results from the P-II study of Lu-177-DGUL in prostate cancer showed that ORR exceeded 80% and 50% reduction in PSA levels of 52.2% of the patients
-
Lu-177-DGUL is developed for the treatment of mCRPC in PSMA+ adult patients who have been unresponsive to standard treatment protocols. CellBion plans to launch Lu-177-DGUL in 2024

Candida Auris Diagnostic Test

-
T2 Biosystems anticipates expanding the test menu to its T2Dx Instrument by adding the C. auris diagnostic test that aims to detect C. auris species directly from blood in just 3-5hrs., without waiting for a positive blood culture
-
This is the company’s 3rd BDD followed by T2Resistance Panel and T2Lyme Panel
-
The company is currently selling T2Candida Panel that runs on fully automated T2Dx Instrument and can detect sepsis-causing fungal pathogens directly from blood without waiting for a positive blood culture
Related Post: https://pharmashots.com/15700/new-drug-designations-june-2023
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














